Create Clarity Across The Publications Lifecycle
Mavens® Scientific Publications Cloud is a Salesforce powered workflow solution purpose-built for publications teams.
See what’s next when you connect the dots with people, data, and AI.

Features and Benefits
Transparent
Increase visibility into key milestones with a configurable timeline view.
Scalable
Enterprise-grade scalability on Salesforce, supporting growth across the organization.
Collaborative
Enable authors and reviewers to collaborate on shared documents in real time.
Integrated
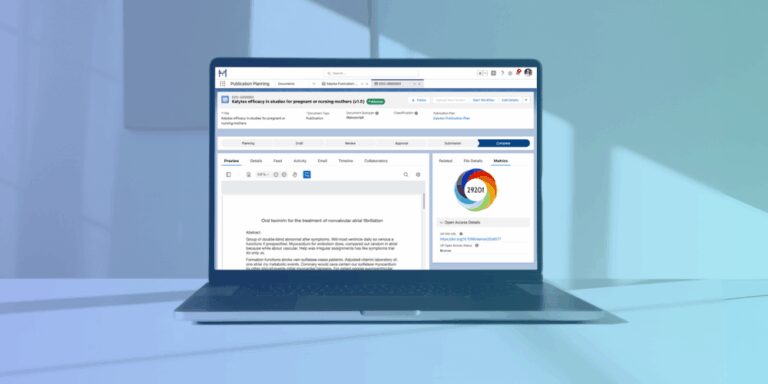
Pre-built integrations include Altmetric for smarter decision-making on journal and congress selection.
Adaptable
Configurable to your SOPs and governance model, with security and role-based access across teams.
AI Powered by Agentforce
Governed AI workflows that speed publications planning, review, and decision-making.
Let’s Connect
Whether you need to modernize Medical Affairs or transform Commercial, our 15+ years of Life Sciences and certified Salesforce experience helps you get it done right, the first time.