Medical Affairs: Product
Publications
Streamline the complete publications process with a solution built for impact.
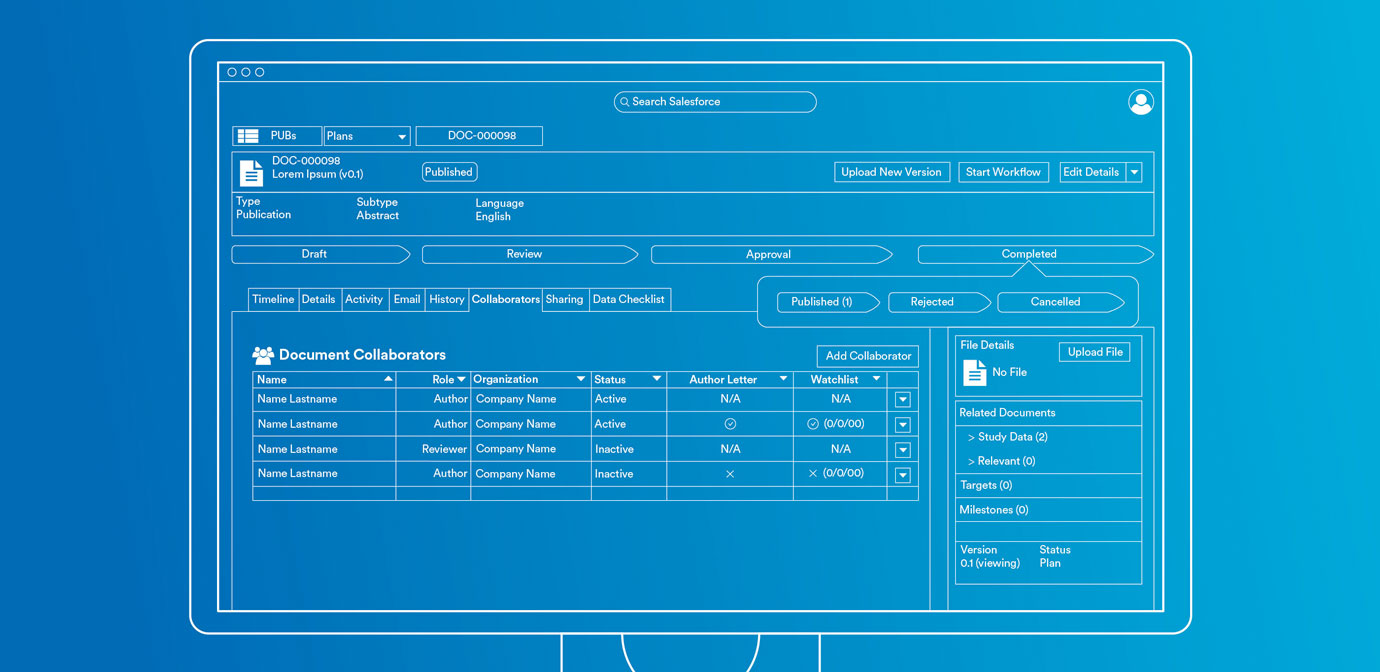
Your complete publication management solution
From study to strategy to publication, Publications ensures every aspect of planning, execution, and analysis are housed in a single solution. Connectors to valuable data sets power informed decision making. Workflows and automation span the entire publication process with actionable data in a single application.
A solution that adapts with your team
Unlike legacy applications that require long build cycles and offer you limited control, Mavens Publications adapts with your team’s quickly evolving needs. It is built on the Salesforce platform with regular releases of new features.
The ability to plan strategically
Data from clinical trials provide a trove of information that can be analyzed and shared for years to cover many aspects of a product and therapeutic area. Teams can align their publication efforts with the scientific imperatives and key medical objectives of a product or franchise. Planning teams can ensure that focus is allocated for the most impact and data is published to reinforce the overall medical strategy.
Benefits
Strategic planning
Align your teams’ publication efforts with key medical objectives, ensuring that publications reinforce the overall medical strategy of a product or therapeutic area.
Data visibility and security
Control data visibility and security. Refine access by therapeutic area, product, or a single document. Predefine roles for your team and assign them manually or automatically as part of a workflow.
Workflows and tasks
Configure workflows by document type. Workflows are flexible, giving the team the ability to adjust and vary the path from one document to the next.
Timeline and milestones
Zoom in and out from a multi-year publication plan to individual tactics and abstract or manuscript progress. Easily update the timeline and let business rules suggest adjustments to milestone changes.
Compliance
Manually and automatically complete compliance checklists as part of a document’s workflow. Author agreements can be configured as prerequisites to viewing a document or data.
Journal and congress selection
Search and compare an integrated database of journals and congresses and save tentative, alternative, and final decisions with a draft. See selections laid out on the timeline to make the best choices for your abstract or manuscript.
Budgets
Manage the administrative and tactical costs for a single publication plan or across a therapeutic area. Maintain rate cards for each agency as well as a reconciliation schedule for each document type.
Author collaboration
Give authors intuitive tools that let them provide input quickly. Track when authors log in or view documents, so the publication management team can monitor progress.
Analytics
Use out-of-the-box reports and create your own with Salesforce reporting tools. Look for trends across a therapeutic area to make informed decisions about how, when, and where to publish.
Native Connectors
Journal/congress database
Search and compare journals and congresses from within the system. Add relevant dates to the timeline.
Reference database
Browse historical publications with a comprehensive Reference Database.
Attention insight system
Understand the reach and influence of your published content.
Electronic signature tool
Finalize contracts from within the system with DocuSign.
See More

Solving Life Sciences toughest challenges.
Our products are powered by Salesforce to provide security and flexibility for the future of Life Sciences.